The micronucleus test according to DIN EN ISO 21427-2 T5 2020-07 or OECD No. 487 is an in vitro method that detects damage to the genetic material and thus a cancer-causing change in the cell. Until now, the method has been limited to documenting the biomarker “micronucleus”, a chromatin-containing structure that occurs in addition to the main nucleus, in cultures of mammalian cells as a consequence of exposure to a test substance. Micronuclei/small nuclei have so far been detected using chromatin staining techniques on fixed cells.
GOBIO has developed an innovative variant of the procedure as an accredited in-house method HM 21427-2, in which micronuclei can be detected on living cells using live image analysis. The method is based on a healthy cell line that expresses a histone 2B green fluorescence fusion protein. As a result, chromatin structures such as micronuclei are visible in the living cell using fluorescence microscopy. The cells used by GOBIO for the micronucleus test are neither derived from tumours nor otherwise immortalized. They have a natural control of the cell cycle and are metabolically competent.
They are capable of natural cell death. Due to these properties, a transformation of the previously healthy cell into a cancer cell can also be tested in addition to the “micronucleus” biomarker. The following changes in nuclear morphology occur when a malignant transformation takes place.
Test execution
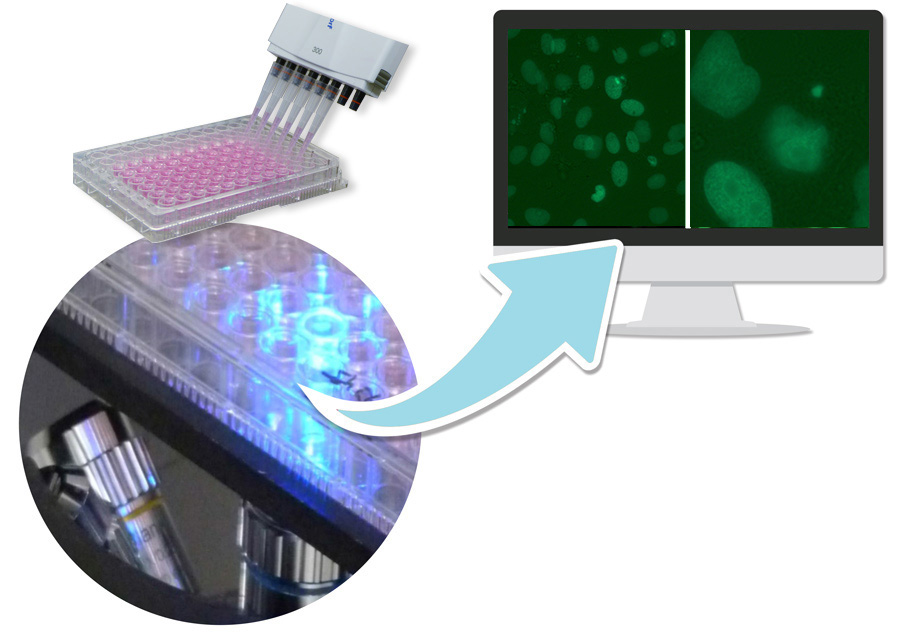
As a rule, a rangefinder test is carried out first to determine cytotoxicity. The test is performed on 96 well plates. The contaminant exposure is carried out in 4 sub-cytotoxic concentrations (8 wells per concentration) when the adherent growing cells have reached a confluence of 60 %. Positive and negative controls are included in each experiment. The micronucleus frequency in test preparations is determined using 2 automatically generated images/per well supported by software. To identify micronuclei, both the fluorescence image and the overlay with the bright field image are analyzed.
At the customer’s request, the measurement can be carried out at different times. This enables the detection of reversible effects, e.g. the reintegration of micronuclei during the interphase.
Please leave your contact details if you would like further information. Link to the contact form.
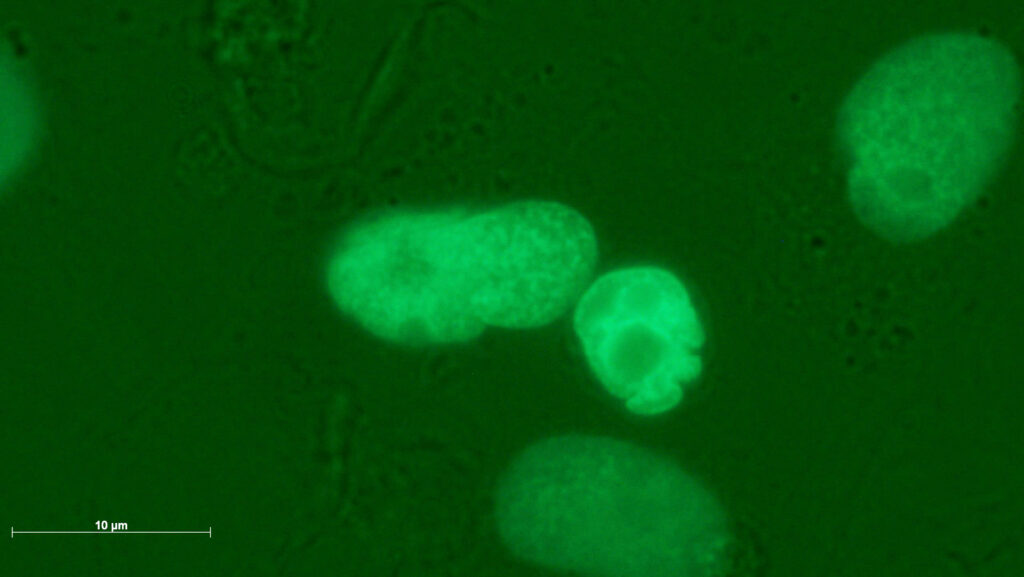
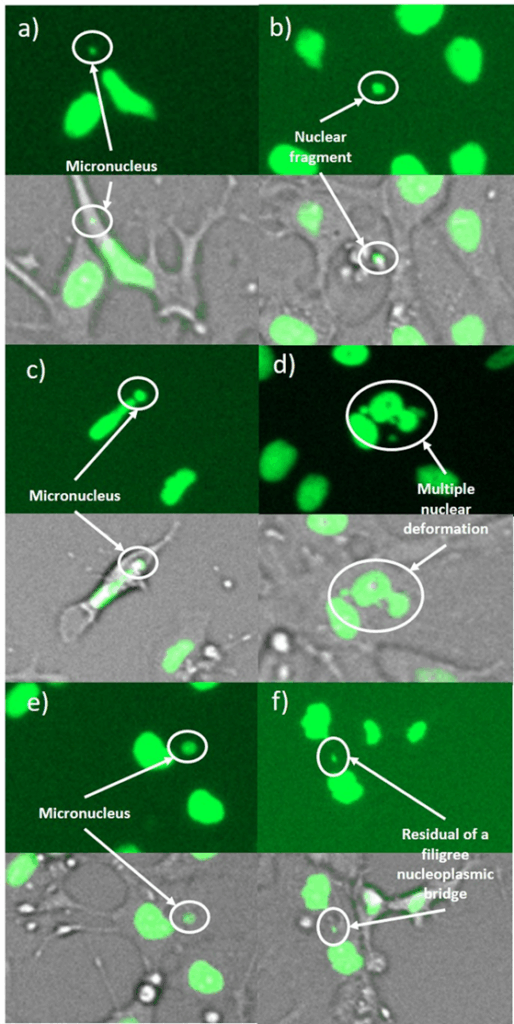
Genotoxic damage pattern with (a,b) micronuclei, (c) a large micronucleus, (d) a multiple deformed nucleus, (e) a nuclear fragment outside a cell and (f) a remnant of a filigree nucleoplasmic bridge. The images were taken in 4-NQO-exposed cultures (a,b,d), in colchicine-exposed cultures (c,e) and in cannabidiol-exposed cultures (f).
The advantages of the new, cost-effective micronucleus test
Peer reviewed
Hölzel, B.N., Pfannkuche, K., Allner, B. et al. Following the adverse outcome pathway from micronucleus to cancer using H2B-eGFP transgenic healthy stem cells. Arch Toxicol 94, 3265-3280 (2020) . For publication.
- Performance characteristics according to DAkkS-approved SOP:
Detection of cytogenetic damage to non-immortalized, healthy cells with functional proliferation control. - The basic functions of the method comply with OECD Guideline No. 487/ DIN EN ISO 21427-2 T5, i.e. the detection of cytogenetic damage that is primarily mitotic in origin. Since the culture, unlike synchronously dividing immortalized cells, also includes long-lived stem cells, damage that occurs during interphase is also reliably recorded.
- The method is mechanism-based. Sub-mechanisms are also mapped
- The test therefore goes beyond the characteristics of OECD Guideline No. 487/ DIN EN ISO 21427-2 T5
- Due to the variety of cellular functions that can be observed in the H2B-GFP-KCB cell line, non-dose-related effects are conceivable, e.g. interference with the genetic basis of motility.
- Therefore, the criterion of dose dependence is reported, but it is not an exclusion criterion for the assessment of genotoxicity.
- Prompt results: 14 days after provision of the test substance/test material
- GLP-compliant test report
- Comprehensive electronic, exposé-capable documentation of the evaluation of the image files
- Cytotoxicity test included at no extra cost
- Metabolic activation of the test substance without animal testing
- Reversibility can be detected by measuring at different points in time
- Further parameters of the health status of the cells can be called up at low cost, e.g. Mitotic index, Motility. Downstream analyses possible: cytokinesis/interphase duration, apoptosis pyknotization, gene expression analyses based on a known expressome.